 Author: Olga Ochneva, Kyrgyzstan
Author: Olga Ochneva, Kyrgyzstan
Hepatitis Prevention Month to commemorate the World Hepatitis Day was organised for the first time in the history of Kyrgyzstan by the Ministry of Health in July this year. Over the recent years, the list of registered and allowed for import medications to treat hepatitis C has been expanded, the new clinical treatment protocol has been approved and a six-year target program to counteract viral hepatitis was adopted. Hepatitis is one of the leading causes of death among people living with HIV and higher-risk populations. Without a doubt, the discussion of availability of hepatitis diagnosis and treatment, introduction of treatment guidelines and implementation of the national viral hepatitis interventions will be an important part of the International AIDS Conference in Amsterdam in 2018. We discussed the reasons for the increased attention to the problem of hepatitis in KyrgyzstanIn with Nurgul Ibraeva, Chief Officer of the Department of Health Services and Medications Policy of the Kyrgyz Ministry of Health.
Statistics and the real picture
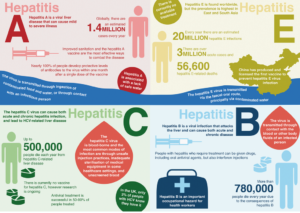
“The problem of viral hepatitis in Kyrgyzstan is growing every year. Blood-borne hepatitis B and C remain a challenging concern, as patients consult the doctors whey they already have advanced illness and complications, such as liver cirrhosis and cancer. During the Hepatitis Prevention Month, we raised awareness in the population about the need to get tested and offered discounted tests that were supported by private laboratories. Many people in Kyrgyzstan find the price for hepatitis testing (around $50) challenging, so patients often discover their disease at an advanced stage,” Nurgul Ibraeva says. “Following the official statistics, in the last five years 11,000–22,000 people with viral hepatitis were registered on an annual basis. Health services provide treatment to more than 2,000 patients with parenteral hepatitis (hepatitis B, C and D – author’s note), but we believe that the actual number of those infected is much higher: more than 250,000 people.”
Prevalence of hepatitis A is the highest. It accounts for 96% of the registered cases, with blood-borne hepatitis B coming second. According to the National Immunization Schedule, since 2000, hepatitis B vaccine is administered to all newborns free-of-charge. As a result, hepatitis B incidence had a fourfold decrease over the last 16 years. Currently, our health services register around 300–400 new cases of hepatitis B among adults annually, while incidence among children dropped to several isolated cases.
“Immunization brings its fruit. According to the Ministry of Health regulation, health workers exposed to blood should be vaccinated, yet no funds are allocated for it, and not every health worker can afford a vaccine,” Nurgul Ibraeva is saying. “Unfortunately, there is no vaccine against hepatitis C. Even if you use means of protection and take the necessary precautions, there is always a risk. Some health care staff remain untested, and it is our estimate that around 1000 health workers have hepatitis C.”
According to the Republican AIDS Center and the Research and Production Association “Preventive medicine” of the Kyrgyz Ministry of Health, in 2014–2015, the share of health personnel with hepatitis C in the general HCV prevalence amounted to 2.5%. The same percentage is attributed to the general public.
Hepatitis C prevalence is the highest among people who inject drugs (PWID). In 2010, 50% of all hepatitis C cases was registered among PWID. By 2015, this share dropped to 35%. Inmates are also among those especially vulnerable to hepatitis C. Over the last six years, 24–53% of all cases were identified in correctional institutions.
“Needle exchange services and opioid substitution therapy are available in Kyrgyzstan, including prisons,” Nurgul Ibraeva is telling. “Prevention programs strive to break the chain of transmission, but the share of infections remains high, even though we managed to stabilize the situation.”
As is the case with other population groups, key populations are still inadequately covered by diagnostic services. According to the official data, from 100 to 200 new cases of hepatitis C are annually registered in Kyrgyzstan. However, the estimated number of people with hepatitis C is much higher: 101,960 cases among the general population and more than 11,000 cases among people who use injecting drugs.
 Availability of treatment
Availability of treatment
In April 2014, the coalition of non-governmental organizations under the initiative of the “Partner Network” Association of Harm Reduction Programs successfully lobbied changes in the Kyrgyz patent legislation. This allowed Kyrgyzstan to import and license generic medications to treat hepatitis C. Currently, a 12-week treatment course on the basis of an officially registered drug costs $615 for a generic and $1500 for the original.
“We have access to several licensed medications produced in China, Egypt and India,” Nurgul Ibraeva says. “If earlier treatment for one patient amounted to $15 000–20 000, today patients can choose medications they can afford. With the expansion of the list of available drugs, producers have been lowering their prices. Yet, patients still have to pay for treatment, which is a challenge for key populations.”
All imported medications have been included in the Essential Medicines List, which is a pre-requisite for the potential state procurement in the future. A Target Program to Address Viral Hepatitis for the period till 2022 has been approved, yet it does not guarantee treatment and does not have financial backing for the planned activities. At the same time, only among people living with HIV, the prevalence of parenteral viral hepatitis exceeds 14%. Over the past six years, the registered number of people with HIV and hepatitis C co-infection increased twofold and reached 701 cases in 2015. Advocates succeeded to include annual hepatitis C treatment for 100 people with HIV into the State Program for HIV Control. Treatment will be financed by the government for the period of five years. Besides, this year a Clinical Protocol for Diagnosis, Treatment and Prevention of Viral Hepatitis B, C and D has been approved. The document is aligned with the latest WHO recommendations and treatment regimens based on direct acting antiviral drugs that are widely available on the market.



